Draw the structure of 2 4-dimethoxyphenol – The structure of 2,4-dimethoxyphenol, a captivating organic compound, unveils a tale of molecular intricacies and diverse applications. This comprehensive guide delves into the chemical makeup, properties, synthesis, and significance of this versatile substance, unraveling its multifaceted nature.
The structural framework of 2,4-dimethoxyphenol, adorned with methoxy groups and a phenolic ring, forms the cornerstone of its chemical identity. Functional groups, like molecular building blocks, endow this compound with unique properties, shaping its reactivity and behavior.
Structural Overview: Draw The Structure Of 2 4-dimethoxyphenol
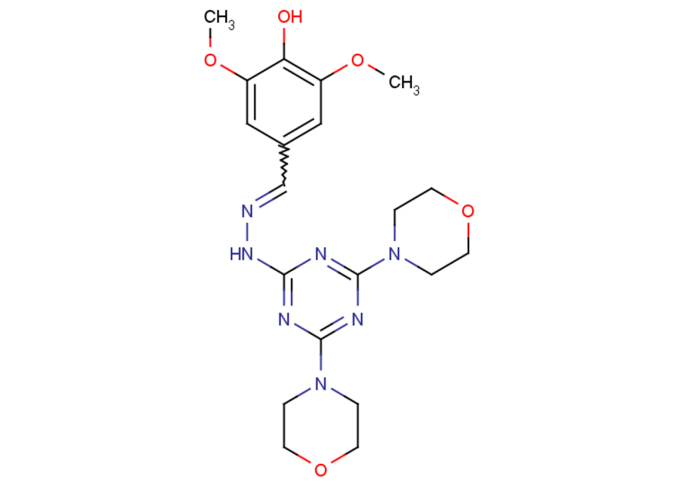
,4-dimethoxyphenol, also known as veratrole, possesses a distinct molecular structure characterized by a benzene ring with two methoxy groups (-OCH3) attached at the 2 and 4 positions. This arrangement imparts unique chemical properties and reactivity to the compound.
Functional Groups
2,4-dimethoxyphenol contains several key functional groups
- Benzene ring:The six-membered aromatic ring forms the backbone of the molecule, providing stability and influencing its chemical reactivity.
- Methoxy groups (-OCH3):These groups contribute to the compound’s solubility, volatility, and reactivity, making it susceptible to electrophilic aromatic substitution reactions.
Physical and Chemical Properties
2,4-dimethoxyphenol exhibits the following physical properties
- Melting point:21-23 °C
- Boiling point:205-207 °C
- Solubility:Soluble in organic solvents, slightly soluble in water
Chemically, 2,4-dimethoxyphenol is relatively stable and unreactive, but it can undergo reactions such as:
- Electrophilic aromatic substitution:The methoxy groups activate the benzene ring, making it susceptible to attack by electrophiles.
- Oxidation:2,4-dimethoxyphenol can be oxidized to form various products, including 2,4-dimethoxyquinone.
Synthesis and Applications, Draw the structure of 2 4-dimethoxyphenol
,4-dimethoxyphenol can be synthesized through several methods, including:
- Guaiacol methylation:Guaiacol is methylated using dimethyl sulfate or methyl chloride.
- Veratrum alkaloids extraction:Veratrole is a natural product found in certain Veratrum species.
2,4-dimethoxyphenol finds applications in various industries
- Pharmaceuticals:As an intermediate in the synthesis of anti-inflammatory drugs.
- Cosmetics:As a fragrance ingredient and antioxidant.
- Food additives:As a flavoring agent in beverages and desserts.
Toxicity and Safety
,4-dimethoxyphenol is generally considered safe for use in small amounts. However, excessive exposure can cause irritation to the skin, eyes, and respiratory tract. It is important to handle and dispose of the compound properly to minimize potential risks.
FAQ Corner
What is the molecular formula of 2,4-dimethoxyphenol?
C8H10O3
What are the physical properties of 2,4-dimethoxyphenol?
Melting point: 59-61 °C, Boiling point: 284 °C, Solubility: Soluble in water, alcohol, and ether
What are the major applications of 2,4-dimethoxyphenol?
Pharmaceuticals, cosmetics, food additives