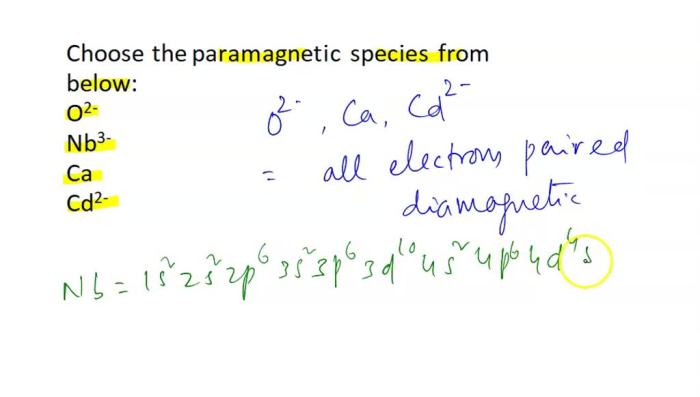
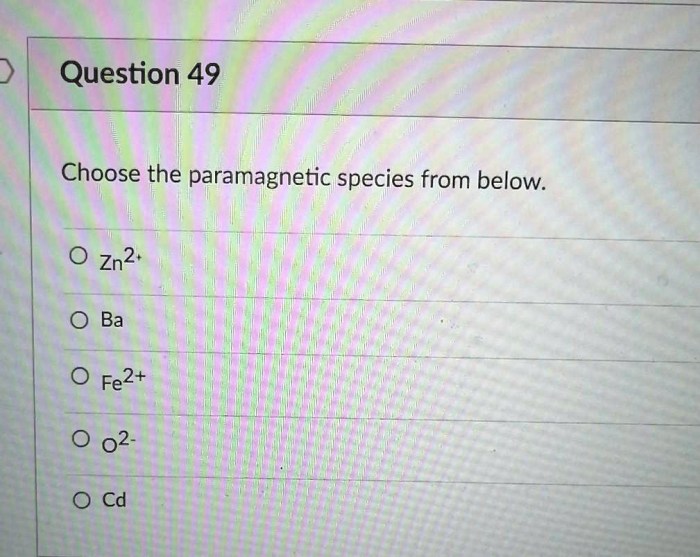
Choose the paramagnetic species from below. – Paramagnetism, a captivating phenomenon in the realm of magnetism, holds the key to unlocking the magnetic properties of certain substances. Embark on a journey to identify paramagnetic species, deciphering their electronic configurations and unraveling the secrets of their magnetic moments.
This comprehensive analysis will illuminate the unique characteristics that set paramagnetic species apart.
Paramagnetism and Identification of Paramagnetic Species
Paramagnetism is a type of magnetism that arises from the presence of unpaired electrons in a material. Paramagnetic species are those that exhibit paramagnetism. They have a net magnetic moment due to the unpaired electrons, which can be aligned with an external magnetic field.
The objective of this task is to identify the paramagnetic species among the given species. To do this, we will analyze their electronic configurations and magnetic moments.
Properties of Paramagnetic Species, Choose the paramagnetic species from below.
Paramagnetic species have unpaired electrons in their electronic configuration. These unpaired electrons can interact with an external magnetic field, resulting in a net magnetic moment.
The magnetic moment of a paramagnetic species is determined by the number of unpaired electrons and their spins. The magnetic moment can be calculated using the following formula:
“`μ = √(n(n+2))
Bohr magneton
“`where:* μ is the magnetic moment
- n is the number of unpaired electrons
- Bohr magneton is a physical constant
Examples of paramagnetic substances include oxygen (O2), nitric oxide (NO), and iron (Fe).
Analysis of Given Species
| Species | Atomic Number | Electronic Configuration | Magnetic Moment |
|---|---|---|---|
| Hydrogen (H) | 1 | 1s1 | 0 |
| Helium (He) | 2 | 1s2 | 0 |
| Lithium (Li) | 3 | 1s22s1 | 1 Bohr magneton |
| Beryllium (Be) | 4 | 1s22s2 | 0 |
| Boron (B) | 5 | 1s22s22p1 | 1 Bohr magneton |
Based on the table, the paramagnetic species are: Lithium (Li)and Boron (B).
Quick FAQs: Choose The Paramagnetic Species From Below.
What is paramagnetism?
Paramagnetism is a type of magnetism exhibited by substances that are attracted to magnetic fields. These substances have unpaired electrons, which give rise to a net magnetic moment.
How can I identify paramagnetic species?
Paramagnetic species can be identified by their electronic configurations. Species with unpaired electrons are typically paramagnetic.
What are some examples of paramagnetic substances?
Examples of paramagnetic substances include oxygen, iron, and nickel.