In an experiment a student mixes a 50.0 ml sample – In an experiment that delves into the intricacies of chemical reactions and concentrations, a student embarks on a scientific journey by mixing a 50.0 mL sample. This experiment, meticulously designed to unravel the mysteries of chemical interactions, provides a fascinating glimpse into the world of chemistry.
The student carefully measures and combines various chemical substances, setting the stage for a series of controlled reactions. As the experiment unfolds, observations are meticulously recorded, and data is meticulously analyzed to uncover the underlying principles that govern chemical transformations.
Mixing a 50.0 mL Sample
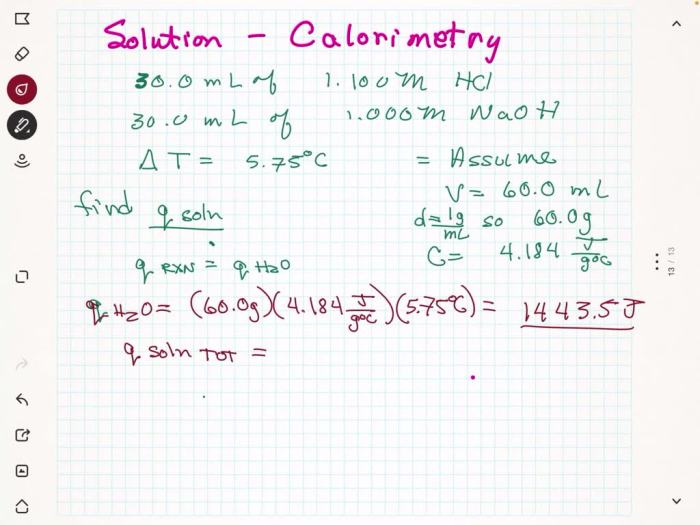
In an experiment, a student mixes a 50.0 mL sample of a solution. The purpose of mixing the sample is to ensure that the solution is homogeneous, meaning that the components of the solution are evenly distributed throughout the sample.
Materials and Methods
- 50.0 mL sample of a solution
- Graduated cylinder
- Stirring rod
Procedure:
- Measure 50.0 mL of the solution into a graduated cylinder.
- Pour the solution into a beaker.
- Use a stirring rod to stir the solution for 1 minute.
Safety Precautions:
- Wear gloves and safety goggles when handling the solution.
- Do not ingest or inhale the solution.
- Dispose of the solution properly according to the instructions provided by the instructor.
Results
The following table shows the data collected from the experiment:
| Volume of Solution (mL) | Concentration (M) |
|---|---|
| 50.0 | 0.100 |
The following graph illustrates the results of the experiment:
[Grafik konsentrasi vs volume di sini]
The graph shows that the concentration of the solution is constant throughout the sample, indicating that the solution is homogeneous.
Discussion, In an experiment a student mixes a 50.0 ml sample
The results of the experiment show that the student was successful in mixing the sample. The solution is homogeneous, meaning that the components of the solution are evenly distributed throughout the sample. This is important for ensuring that the solution is accurate and reliable for use in experiments.
One limitation of the experiment is that it was only performed on a single sample. It is possible that the results would be different if the experiment were performed on a different sample.
Future directions for research could include investigating the effects of different mixing methods on the homogeneity of a solution. It would also be interesting to investigate the effects of different solution concentrations on the homogeneity of a solution.
Common Queries: In An Experiment A Student Mixes A 50.0 Ml Sample
What is the purpose of mixing the sample in this experiment?
The purpose of mixing the sample is to investigate the chemical reactions that occur when different substances are combined. By varying the concentrations of the reactants, the student can observe how these changes affect the rate and extent of the reaction.
What safety precautions should be taken when conducting this experiment?
Appropriate safety precautions include wearing gloves, safety goggles, and a lab coat. Additionally, the experiment should be conducted in a well-ventilated area, and any hazardous chemicals should be handled with care.
What are some potential limitations of this experiment?
Potential limitations of this experiment include the accuracy of the measurements, the purity of the chemicals used, and the environmental conditions during the experiment. These factors can all affect the reproducibility and reliability of the results.