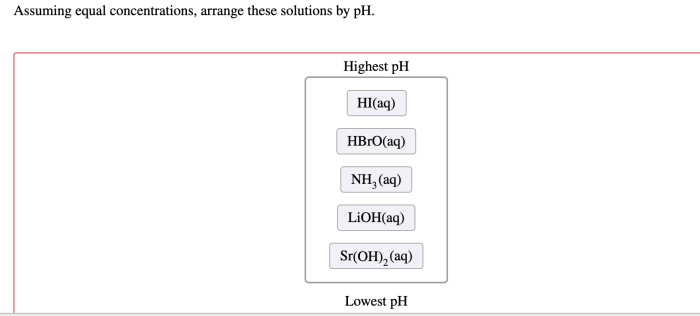
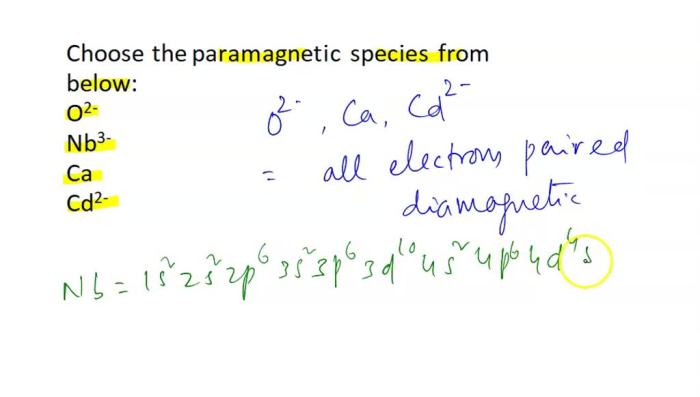
Cracking the periodic table code is an intriguing endeavor that unveils the hidden patterns and relationships within the elements, providing a powerful tool for understanding and predicting their properties and behavior.
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number, atomic mass, and electron configuration. This arrangement reveals periodic trends in properties such as atomic radius, ionization energy, and electronegativity, enabling chemists to make informed predictions about an element’s chemical reactivity and bonding behavior.
Introduction to the Periodic Table
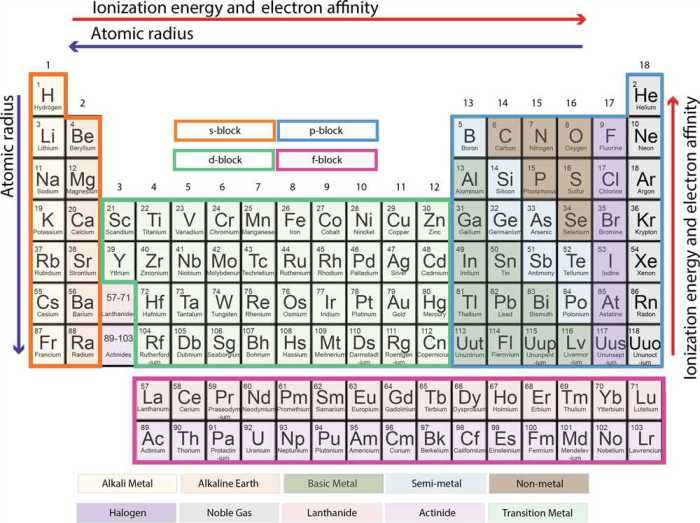
The periodic table is a tabular arrangement of chemical elements, organized on the basis of their atomic number, electron configurations, and recurring chemical properties. It is a powerful tool that provides a wealth of information about the elements and their behavior.The
periodic table consists of 18 vertical columns, called groups, and 7 horizontal rows, called periods. The elements are arranged in the table in order of increasing atomic number, which is the number of protons in the nucleus of an atom.
The atomic number of an element uniquely identifies it and determines its position in the periodic table.The periodic table is a valuable resource for chemists and other scientists. It can be used to predict the properties of an element based on its position in the table, and to understand the chemical reactions that an element can undergo.
Understanding the Patterns in the Periodic Table
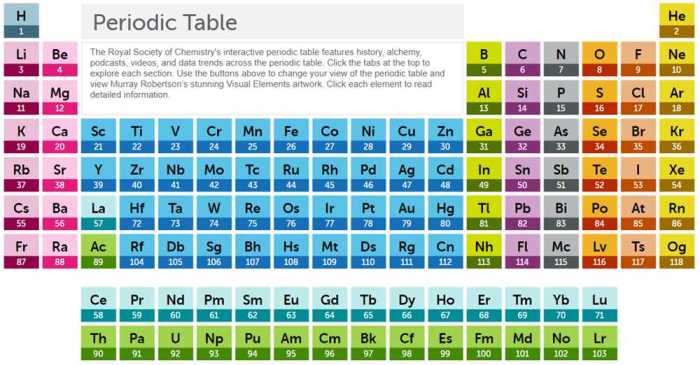
The periodic table is a tabular arrangement of chemical elements, organized on the basis of their atomic number, electron configuration, and recurring chemical properties. It allows us to identify patterns and trends in the properties of elements, which can be used to predict their chemical behavior.
The periodic table is divided into horizontal rows, called periods, and vertical columns, called groups. Elements in the same period have the same number of electron shells, while elements in the same group have the same number of valence electrons.
These arrangements lead to periodic trends in the properties of the elements.
Periodic Trends
Several periodic trends can be observed in the periodic table, including:
- Atomic radius:The atomic radius generally increases down a group and decreases across a period. This is because the number of electron shells increases down a group, leading to a larger atomic radius, while the effective nuclear charge increases across a period, leading to a smaller atomic radius.
- Ionization energy:Ionization energy is the energy required to remove an electron from an atom. It generally increases across a period and decreases down a group. This is because the effective nuclear charge increases across a period, making it more difficult to remove an electron, while the atomic radius increases down a group, making it easier to remove an electron.
- Electronegativity:Electronegativity is the ability of an atom to attract electrons. It generally increases across a period and decreases down a group. This is because the effective nuclear charge increases across a period, making it more electronegative, while the atomic radius increases down a group, making it less electronegative.
Relationship between Position and Reactivity
The position of an element in the periodic table can provide insights into its chemical reactivity. Elements in the same group tend to have similar chemical properties, as they have the same number of valence electrons. For example, all alkali metals (Group 1) are highly reactive and form 1+ ions.
Elements in the same period tend to have different chemical properties, as they have different numbers of valence electrons. However, there are some general trends. For example, elements on the left side of a period are typically metals, while elements on the right side are typically non-metals.
Additionally, the reactivity of metals generally decreases down a group, while the reactivity of non-metals generally increases down a group.
Decoding the Periodic Table for Element Properties
The periodic table is a powerful tool for predicting the properties of elements based on their position. This is because the elements are arranged in a way that reflects their atomic structure and chemical behavior.By understanding the patterns in the periodic table, we can determine an element’s valence electrons, oxidation states, and chemical bonding behavior.
This information can then be used to predict the element’s physical and chemical properties.
Valence Electrons
The valence electrons of an element are the electrons in its outermost energy level. These electrons are responsible for the element’s chemical bonding behavior. The number of valence electrons can be determined by looking at the element’s group number in the periodic table.
Elements in the same group have the same number of valence electrons.For example, all of the elements in Group 1 have one valence electron. This means that they are all highly reactive and form ionic bonds with other elements.
Oxidation States
The oxidation state of an element is the charge that it has when it forms chemical bonds. The oxidation state of an element can be determined by looking at its position in the periodic table. Elements in the same period have the same number of energy levels.
The number of energy levels determines the maximum oxidation state of the element.For example, all of the elements in Period 2 have two energy levels. This means that they can have a maximum oxidation state of +2 or
2.
Chemical Bonding Behavior
The chemical bonding behavior of an element is determined by its valence electrons and its oxidation state. Elements with similar valence electrons and oxidation states tend to form similar types of chemical bonds.For example, all of the elements in Group 1 have one valence electron and a +1 oxidation state.
This means that they all form ionic bonds with other elements.
Exceptions and Limitations
There are some exceptions and limitations to using the periodic table to predict element properties. For example, some elements have variable oxidation states. This means that they can form different types of chemical bonds with different elements.Additionally, the periodic table does not take into account the effects of temperature and pressure on element properties.
These factors can also affect the way that elements behave chemically.Despite these limitations, the periodic table is a valuable tool for understanding the properties of elements and predicting their chemical behavior.
Historical Development of the Periodic Table
The periodic table is a tabular arrangement of chemical elements, organized on the basis of their atomic number, electron configuration, and recurring chemical properties. Its development has been a collaborative effort of many scientists over centuries.
Mendeleev’s Contribution
In 1869, Dmitri Mendeleev published the first widely recognized periodic table, which arranged the known elements in order of increasing atomic mass. Mendeleev’s table showed that elements with similar chemical properties recurred at regular intervals, forming vertical columns known as groups.
He also predicted the existence of several new elements, which were later discovered and confirmed his theory.
Moseley’s Contribution
In 1913, Henry Moseley discovered that the atomic number of an element, rather than its atomic mass, was the fundamental property that determined its position in the periodic table. Moseley’s discovery led to a more accurate arrangement of the elements and helped to establish the modern periodic table.
Discovery of New Elements
The discovery of new elements throughout the 20th century led to the expansion of the periodic table. These new elements, such as the noble gases and the transuranium elements, helped to fill in gaps in the table and refine our understanding of the periodic law.
Advancement of Atomic Theory
The development of atomic theory, particularly the understanding of electron configuration, played a crucial role in the refinement of the periodic table. The arrangement of electrons in atomic orbitals explained the chemical properties of elements and helped to justify the periodic trends observed in the table.
Role in the Evolution of Chemistry
The periodic table has been a central organizing principle in chemistry, providing a framework for understanding the behavior of elements and their interactions. It has been used to predict the properties of new elements, develop new materials, and deepen our understanding of chemical bonding and reactivity.
Applications of the Periodic Table in Chemistry: Cracking The Periodic Table Code
The periodic table is a powerful tool for organizing and understanding chemical information. It is used to classify elements, predict chemical reactions, and design new materials.
One of the most important applications of the periodic table is in the classification of elements. The periodic table groups elements with similar chemical properties together, making it easy to identify and compare their properties. For example, all of the alkali metals (Group 1) are highly reactive and form 1+ ions, while all of the halogens (Group 17) are highly reactive and form 1- ions.
Predicting Chemical Reactions, Cracking the periodic table code
The periodic table can also be used to predict chemical reactions. By looking at the position of an element on the periodic table, it is possible to predict its reactivity and the types of reactions it will undergo. For example, elements in the same group tend to react in similar ways.
This knowledge can be used to predict the products of a chemical reaction.
Designing New Materials
The periodic table is also a valuable tool for designing new materials. By understanding the properties of different elements, it is possible to create materials with specific properties. For example, the periodic table has been used to design new alloys, semiconductors, and polymers.
Applications in Various Fields of Chemistry
The periodic table is used in a wide variety of fields of chemistry, including inorganic chemistry, organic chemistry, and biochemistry. In inorganic chemistry, the periodic table is used to classify elements and predict their chemical properties. In organic chemistry, the periodic table is used to understand the structure and reactivity of organic molecules.
In biochemistry, the periodic table is used to understand the role of elements in biological systems.
Limitations of the Periodic Table
While the periodic table provides a valuable framework for understanding the properties of elements, it has certain limitations in predicting the properties of certain elements, particularly transition metals and lanthanides.
Factors Not Accounted For
The periodic table does not account for factors such as atomic orbitals, molecular bonding, and quantum effects, which can significantly influence the properties of elements. For instance, transition metals exhibit variable oxidation states and can form complex ions due to their partially filled d orbitals.
Lanthanides, on the other hand, have similar chemical properties due to the presence of f electrons, which are not adequately represented in the periodic table.
Ongoing Research and Refinement
Despite its limitations, the periodic table remains a fundamental tool in chemistry. Ongoing research is focused on refining and extending the periodic table to better account for the complexities of element properties. This includes exploring new ways to represent atomic orbitals and molecular bonding, as well as investigating the behavior of elements in extreme conditions.
Commonly Asked Questions
What is the significance of the periodic table in chemistry?
The periodic table is a systematic arrangement of elements that reveals periodic trends in their properties, enabling chemists to predict element behavior and reactivity.
How can the periodic table be used to predict element properties?
The position of an element in the periodic table provides valuable clues about its atomic number, electron configuration, and chemical properties, allowing chemists to make informed predictions about its valence electrons, oxidation states, and bonding behavior.
What are the limitations of the periodic table?
While the periodic table is a powerful tool, it has limitations in predicting the properties of certain elements, such as transition metals and lanthanides. It does not account for factors like atomic orbitals, molecular bonding, and quantum effects, which can influence element behavior.